On June 6, 2023, the Patented Medicine Prices Review Board (PMPRB) released its first NEWSletter of 2023, providing updates on recent activities among the Board and Board staff. Updates from the NEWSletter and PDCI’s insights on potential implications are summarized below. Despite recent quietness from PMPRB on its plans for guidelines development, it’s clear that PMPRB is continuing to conduct business under its interim guidelines and will process open investigations accordingly.
- Meetings: The Board and Board staff held their first meeting of 2023 in Vancouver, BC on March 29, 2023 where the path forward for the regulatory framework modernization was discussed. A second meeting of the Board and Board staff was held in Nova Scotia on June 7, 2023.
- PMPRB Staffing Update: Douglas Clark’s last day as Executive Director was June 1, 2023. Guillaume Couillard has been appointed Acting Executive Director until Mr. Clark’s permanent successor is in place.
- Continuation of Interim Guidelines: As published in the August 2022 Interim Guidance, the June 2023 NEWSletter restated that any patented medicines that have not received a Maximum Average Potential Price (MAPP) or Non-Excessive Average Price (NEAP) prior to July 1, 2022, will not be subject to a review by PMPRB staff until new Guidelines are in force.
- 2022 Investigations: PMPRB indicated in its August 2022 Interim Guidance it would commence an investigation if any of the following criteria were met:
- National Average Transaction Price (N-ATP) of a medicine exceeded its N-NEAP projected in the compliance report resulting in more than $50,000 of excess revenues
- A list price increased by more than the allowable CPI during the January to June 2022 reporting period
- A list price increased during the July to December 2022 reporting period
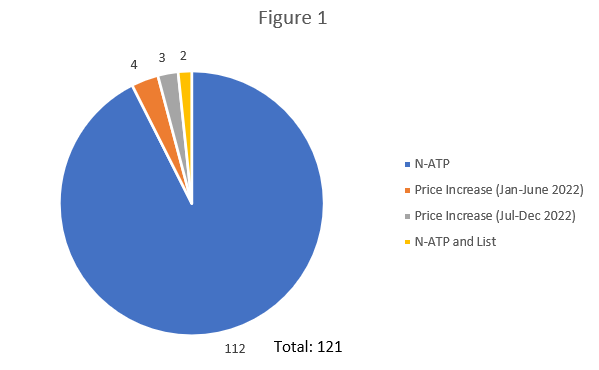
A total of 121 patented medicines met the above criteria resulting in investigations being commenced. Per Figure 1 below, the majority were related to N-ATP as opposed to price increases. This number is somewhat lower than previous years, due partly to the inability for international prices to be used as a compliance factor.
- Of note, two of these investigations were closed after Voluntary Compliance Undertakings (VCUs) were accepted by the new Board Chair, Thomas Digby – his first in his new role.
- On the issue of list price increases, the August 2022 Interim Guidance indicated this would be revisited if the interim period extended into 2023, however PMPRB has yet to publicly clarify whether list price increases in 2023 will trigger investigation.
- Human Drug Advisory Panel Membership Update: While the future role of PMPRB’s scientific review panel remains unclear, membership continues to evolve: Dr. Bishal Gyawali attended the May 2023 HDAP meeting intended for resubmissions. Dr. Gyawali replaces outgoing member Dr. Fred Aoki who had served on the HDAP since 2009.
For more information, please consult PMPRB’s June 2023 NEWSletter or contact Dylan Lamb-Palmer, PDCI’s Associate Director, Pricing and Data Analytics at dylan.lambpalmer@pdci.ca for any questions about future directions of PMPRB.
