Each month the pCPA issues an update on the previous month’s negotiation activity. Below, you will find an overview of the new pCPA activity along with PDCI insights into recent developments for your interest.
| Summary of New pCPA Activity Since Last Update | ||
|
Category
|
New Files
|
Total Files
|
|
Active Negotiations
|
2
|
|
| Completed Negotiations (with LOI) | 6 | 287 |
| Completed with No Agreement (with no LOI) | 2 | 47 |
| No Negotiations | 1 | 69 |
| Consideration at P/T Level | 0 | 13 |
PDCI TARGET INSIGHTS (Since Last pCPA Update)
Active Negotiations:
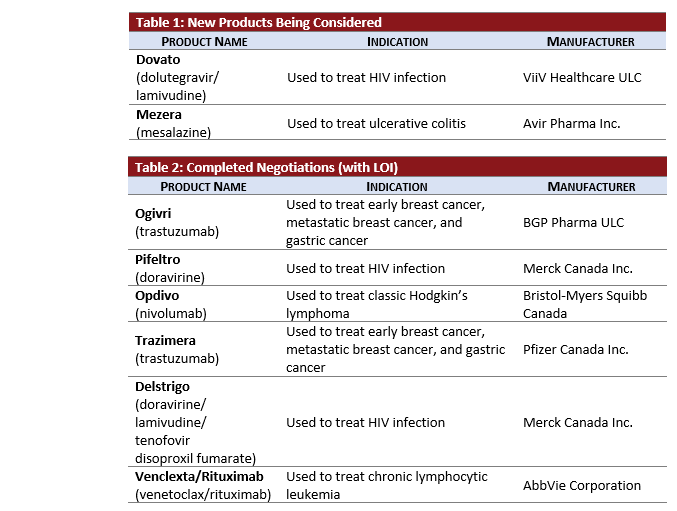
- MEZERA, a new 5-ASA product and likely considered a line extension, did not complete a CDR review but entered pCPA negotiations in October subsequent to a positive recommendation from INESSS in August 2018.
- DOVATO is in active negotiations 1-month after obtaining its CDR Recommendation.
Completed Negotiations:
- 3 Oncology products’ negotiations for breast cancer were completed in this month’s update:
- OGIVRI and TRAZIMERA: biosimilars of trastuzumab achieved LOI in October.
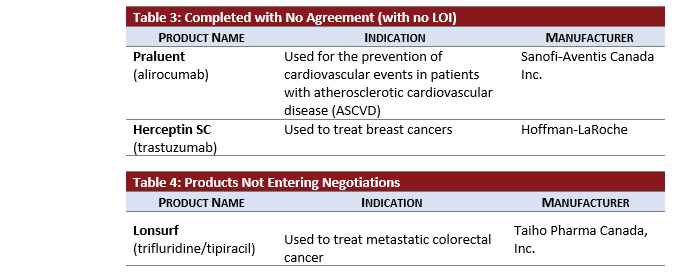
- HERCEPTIN SC (subcutaneous trastuzumab) negotiations completed without an agreement following negotiations that began in January 2019.
- 2 HIV products manufactured by Merck, DELSTRIGO (doravirine) and PIFELTRO (doravirine, lamivudine, tenofovir disoproxil fumarate) and reviewed in parallel at CDR, completed negotiations together in 4 months.
Other Considerations:
- LONSURF received a previous negative reimbursement recommendation from pCODR in July 2018. Following a resubmission recommendation to not reimburse in September 2019, the pCPA added LONSURF for colorectal cancer to the “No Negotiations” list in October 2019. However, LONSURF was listed on the completed LOI list in August 2019 following INESSS advice to the Minister in May 2018.
PDCI TARGET TRENDS

To find out more information about pCPA negotiations and how PDCI’s reimbursement strategy team can help navigate through pCPA discussions, please contact Kaitlyn Proulx at kaitlyn.proulx@pdci.ca.