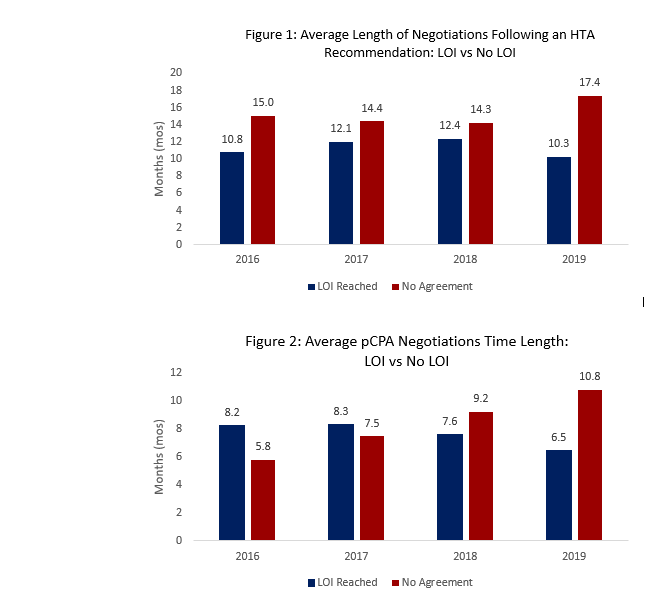
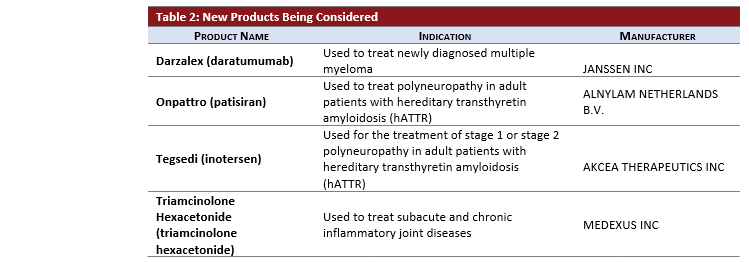
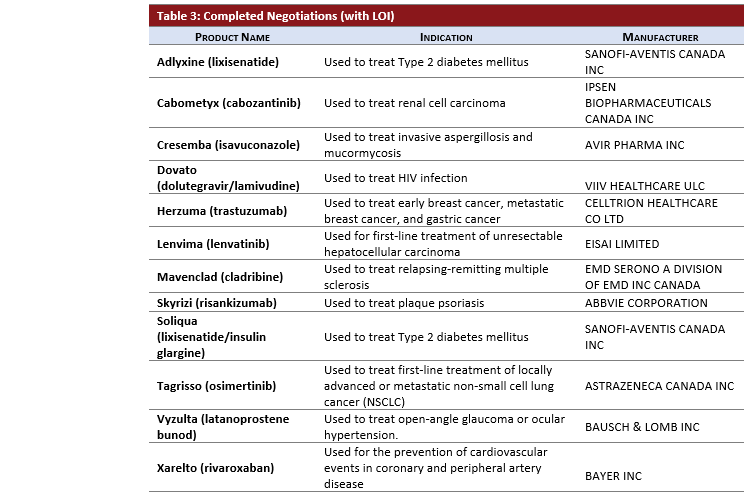
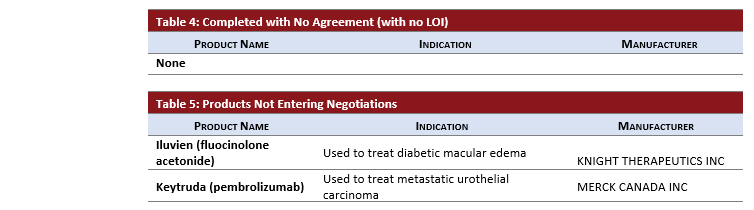
Each month the pCPA issues an update on the previous month’s negotiation activity. Below, you will find an overview of the new pCPA activity along with PDCI insights into recent developments for your interest.
| Table 1. Summary of New pCPA Activity Since Last Update | ||
| Category | New Files | Total Files |
| Active Negotiations | 4 | 34 |
| Completed Negotiations (with LOI) | 12 | 294 |
| Completed with No Agreement (with no LOI) | 0 | 47 |
| No Negotiations | 2 | 72 |
| Consideration at P/T Level | 0 | 13 |
PDCI TARGET INSIGHTS (Since Last pCPA Update)
Active Negotiations:
- 4 products entered active negotiations:
- TEGSEDI and ONPATTRO, both products indicated for treatment of polyneuropathy in adult patients with hereditary transthyretin amyloidosis (hATTR).
- TEGSEDI received INESSS recommendation on September 11, 2019; CDR recommendation is pending (embargoed to December 11).
- ONPATTRO received CADTH recommendation on July 29, 2019.
- Triamcinolone Hexacetonide for treatment of subacute and chronic inflammatory joint diseases.
- DARZALEX for a new indication in multiple myeloma.
- TEGSEDI and ONPATTRO, both products indicated for treatment of polyneuropathy in adult patients with hereditary transthyretin amyloidosis (hATTR).
Completed Negotiations:
- With a total of 12 drug products completing negotiations with an LOI, the pCPA concluded a record number of LOIs in November.
- HERZUMA an oncology biosimilar to HERCEPTIN completed negotiations with an LOI reached.
Other Considerations:
- After receiving negative CADTH recommendations, KEYTRUDA and ILUVIEN did not enter negotiations.
PDCI TARGET TRENDS