Each month the pCPA issues an update on the previous month’s negotiation activity. Below you will find an overview of the new pCPA activity along with PDCI insights into recent developments for your interest.
| Summary of New pCPA Activity Since Last Update | ||
| Category | New Files | Total Files |
| Active Negotiations | 9 | 49 |
| Completed Negotiations (with LOI) | 4 | 257 |
| Completed with No Agreement (no LOI) | 3 | 40 |
| No Negotiations | 1 | 66 |
| Consideration at P/T Level | 0 | 13 |
PDCI Target Insights (since last pCPA Update)
Active Negotiations:
- Two new products for the treatment of HIV entered negotiations in June: DELSTRIGO and PIFELTRO
Completed Negotiations:
- Five of nine products are Oncology treatments (CABOMETYX, FOLOTYN, ONIVYDE, TAFINLAR/MEKINIST, VENCLEXTA/RITUXIMAB)
- BIKTARVY completed negotiations for HIV treatment
- SPINRAZA (used to treat spinal muscular atrophy) resubmission completed negotiations
- After almost 3 years on the active list, XOLAIR negotiations ended with no agreement.
PDCI Target pCPA Trends
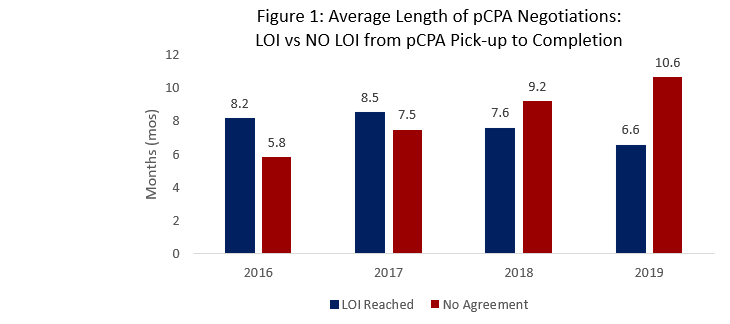
- The average length of pCPA negotiations is continuing to shorten for successful LOIs versus prior years, averaging 6.6 months for 2019 (Figure 1). A trend is emerging with shorter times to positive agreements versus longer times to no agreement (averaging 10.6 months in 2019).
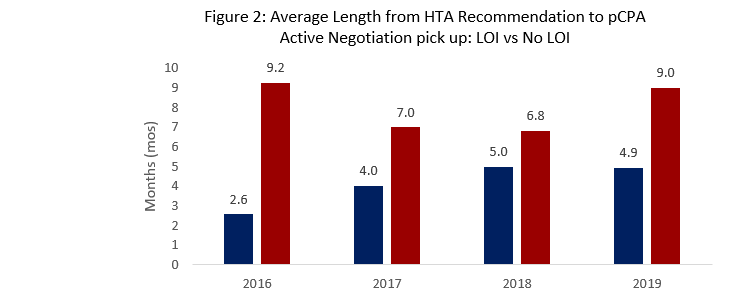
- When evaluating products completing negotiations in 2019, PDCI noted successful LOIs following HTA recommendations are associated with shorter times to pCPA pick-up (4.9 months) versus negotiations that end in no agreement (9 months).
- For 2019 Year-to-date, the average time from HTA Recommendation to pCPA pick-up for all products is shorter (3.3 months) compared to previous years (averaging 5-7 months)
To find out more information about pCPA negotiations and how PDCI’s reimbursement strategy team can help navigate through pCPA discussions, please contact John-Paul Dowson at jp.dowson@pdci.ca