Each month the pCPA issues an update on the previous month’s negotiation activity. Below, you will find an overview of the new pCPA activity along with PDCI insights into recent developments for your interest.
|
Summary of New pCPA Activity Since Last Update |
||
|
Category |
New Files |
Total Files |
| Active Negotiations | 7 | 47 |
| Completed Negotiations (with LOI) | 6 | 263 |
| Completed with No Agreement (with no LOI) | 2 | 43 |
| No Negotiations | 2 | 67 |
| Consideration at P/T Level | 0 | 13 |
PDCI Target Insights (Since Last pCPA Update)
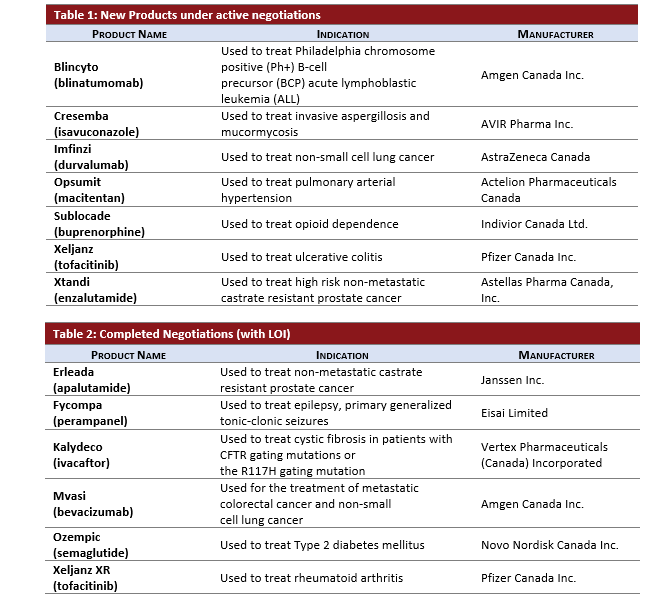
Active Negotiations Considerations:
- 3 Oncology products entered negotiations, BLINCYTO, IMFINZI, XTANDI.
- BLINCYTO for a third indication.
- XTANDI for high risk non-metastatic castrate resistant prostate cancer.
- IMFINZI for non-small cell lung cancer.
Completed Negotiations with LOI:
- MVASI (biosimilar for AVASTIN) is the first oncology biosimilar to complete pCPA negotiations.
- FYCOMPA completed negotiations with approximately 39.5 months after its CDR recommendation in 2016.
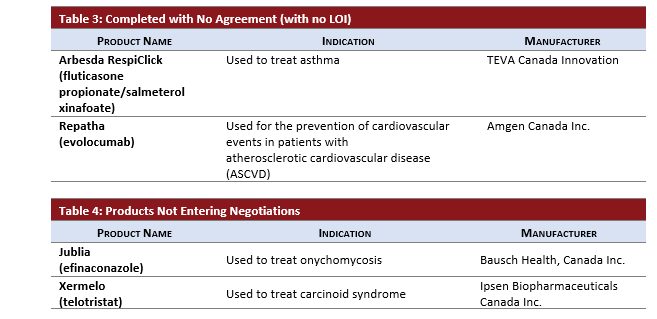
No LOI or No Negotiations:
- REPATHA completed negotiations without an LOI after approximately 20.2 months after an HTA recommendation based on a price reduction of at least 90%.
- ARBESDA RESPICLICK completed negotiations without an LOI after approximately 7.4 months following HTA recommendation.
- JUBLIA and XERMELO are not entering pCPA negotiations after receiving negative CADTH recommendations.
PDCI Target pCPA Trends
To find out more information about pCPA negotiations and how PDCI’s reimbursement strategy team can help navigate through pCPA discussions, please contact John-Paul Dowson at jp.dowson@pdci.ca