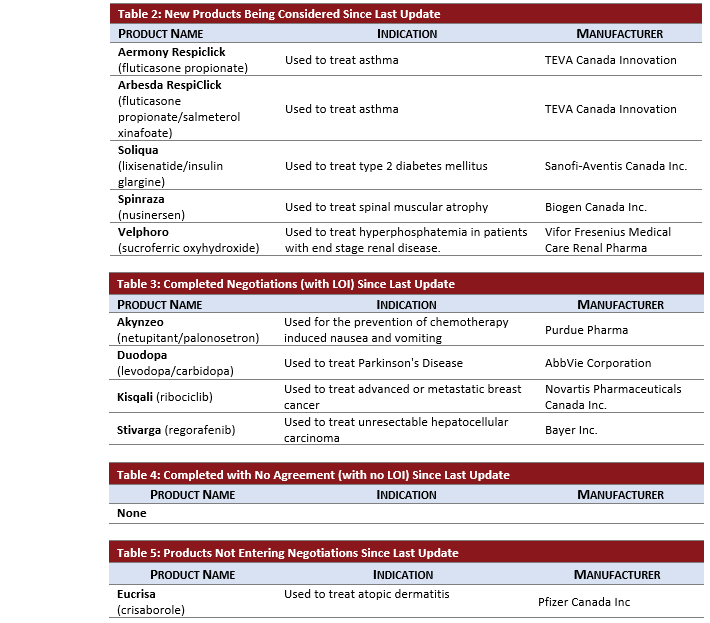
Each month the pCPA issues an update on the previous month’s negotiation activity. In Table 1 below you will find an overview of the new pCPA activity along with PDCI insights into recent developments for your interest.
PDCI Target Insights (since last pCPA update)
- pCPA negotiations were initiated in April for:
- Teva’s Aermony Respiclick (fluticasone propionate) and Arbesda RespiClick (fluticasone propionate/salmeterol xinafoate) for maintenance treatment of asthma. These negotiations follow CDR recommendations in December to reimburse, provided costs savings are provided for drug plans.
- Sanofi-Genzyme’s Soliqua (lixisenatide/insulin glargine) used to treat type 2 diabetes mellitus is added to the active list alongside the monotherapy version of lixisenatide, Adlyxine, which has been on the active negotiation list since April 2018.
- Biogen’s Spinraza (nusinersen) was added following a recent CDR review for expanded criteria in Spinal Muscular Atrophy. Spinraza previously completed pCPA negotiations in September 2018.
- Vifor Fresenius’ Velphoro (sucroferric oxyhydroxide) was added for treatment of hyperphosphatemia in patients with end stage renal disease. This is the first time Velphoro has appeared on the pCPA list
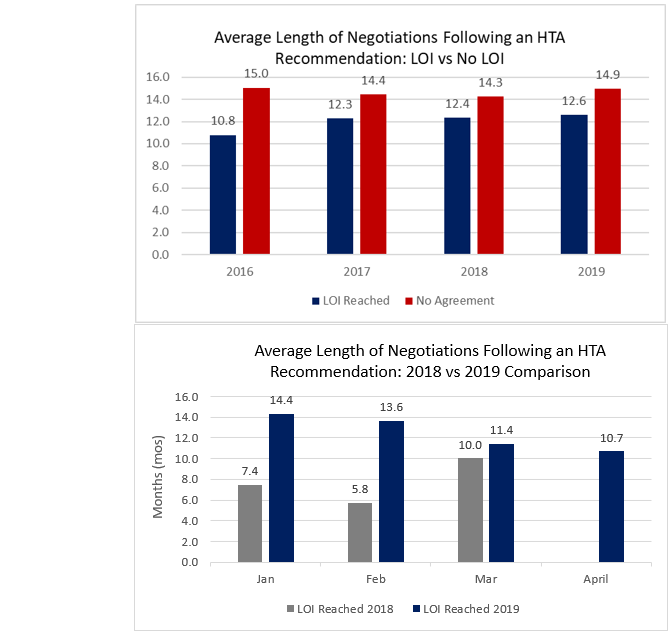
PDCI Target pCPA Trends
- The average time to LOI and No LOI from HTA recommendation is trending at an average length consistent yet marginally higher versus prior years.
- In 2019, the average time to LOI from an HTA recommendation is 12.6 months. In both March and April, pCPA concluded LOIs beating the year-to-date average.
- The 2019 year-to-date average time from HTA recommendation to No LOI is 14.9 months.
- Six products remaining on the active list now exceed the average 12.6 months to LOI benchmark: Fycompa, Viacoram, Kuvan, Xolair, Adlyxine, and Trulicity.
To find out more information about pCPA negotiations and how PDCI’s reimbursement strategy team can help navigate through pCPA discussions, please contact John-Paul Dowson at jp.dowson@pdci.ca