New “PROVISIONAL ALGORITHMS” at the Centre of Changes to Cancer Therapy Reviews
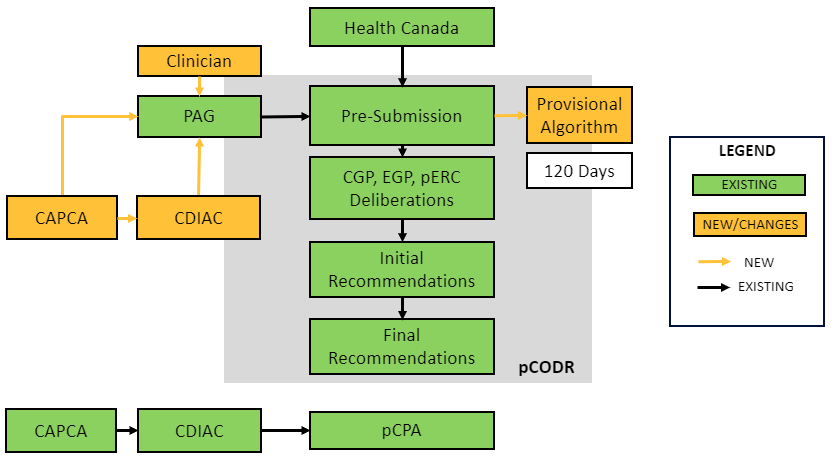
Effective July 1, 2019, process and operational changes will be made to integrate the functions of the Cancer Drug Implementation Advisory Committee (CDIAC) to CADTH and its pCODR process. Solicitation of stakeholder feedback began in March of this year. The integration of CDIAC into the pCODR process is intended to further enhance transparency of cancer therapy review processes and support more informed decision-making. The changes apply to all new pre-submission information forms submitted on or after July 1.
The CDIAC role, which was housed under the Canadian Association of Provincial Cancer Agencies (CAPCA), is to provide advice concerning how new drugs can be integrated into existing funding algorithms to achieve greater consistency in drug funding decisions across Canada. CDIAC historically provided advice after pCODR reviews and prior to pCPA negotiations. The new changes shift its role much earlier, beginning with the pre-submission process of pCODR.
Visual Summary of Changes
Stated Objectives of CDIAC Transition into pCODR
- Enhance transparency of pCODR
- Streamline and reduce duplicative administration processes between pCODR and CAPCA
- Increase stakeholder input into the development of a provisional algorithm for new cancer drugs and indications to:
- Indicate how therapy could be used in comparison to existing funded treatments;
- Consider how the introduction of a new therapy may affect the sequencing of other therapies treating the same indication.
- Increase stakeholder input to
- Understand views and treatment implications of reimbursing a new cancer therapy
- Consider of any issues that may arise through introducing a new cancer therapy into the sequence of already-funded therapies
- Better support jurisdictional decision-making for drug reimbursement
Highlights of Changes to pCODR Resulting from CDIAC Integration
- Expansion of Provincial Advisory Group (PAG) to include CAPCA and two clinician members
- No compositional change to pERC or Clinical Guidance Panels
- Pre-submission information must be provided by submitters minimum 120 calendar days before anticipated filing of complete pCODR submission
- Submitters must provide, during the pre-submission, all relevant comparators that have received an initial or final pERC recommendation, are undergoing negotiations through the pCPA or is publicly funded (including case-by-case funding)
- 1- hour pre-submission meetings will be schedule by teleconference or can be requested in-person. In-person meetings are limited to one meeting within a six-month period.
- Inclusion of provisional algorithm does not presuppose the outcome of reimbursement recommendation by pERC and does not bind participating jurisdictions to fund the new therapy
To further understand implementation issues and delivery of healthcare considerations, an ad hoc clinical panel may be established and/or an online survey may be distributed to clinical leads affiliated with provincial cancer agencies to support development of provisional algorithm. Read CADTH-announced changes here.
