Linking RWD/RWE to Market Access Throughout the Product Lifecycle
At every stage of the market access pathway for drugs and medical technologies, stakeholders increasingly value real-world data (RWD) and real-world evidence (RWE). From filling important evidence gaps in regulatory and health technology assessment (HTA) submissions to demonstrating safety and effectiveness in the real-world to providing key outcomes data to payers, RWD/RWE is playing a key role in market access – and that is only expected to grow.
At McKesson and PDCI, we are experts in both market access and RWD/RWE. Throughout the product lifecycle, we assess our clients’ RWD/RWE needs from a market access perspective, so that the right data is collected and/or analyzed to help achieve their market access goals and advance health outcomes for all. Our comprehensive RWD/RWE services are shown in Figures 1 and 2 below and include the following significant offerings:
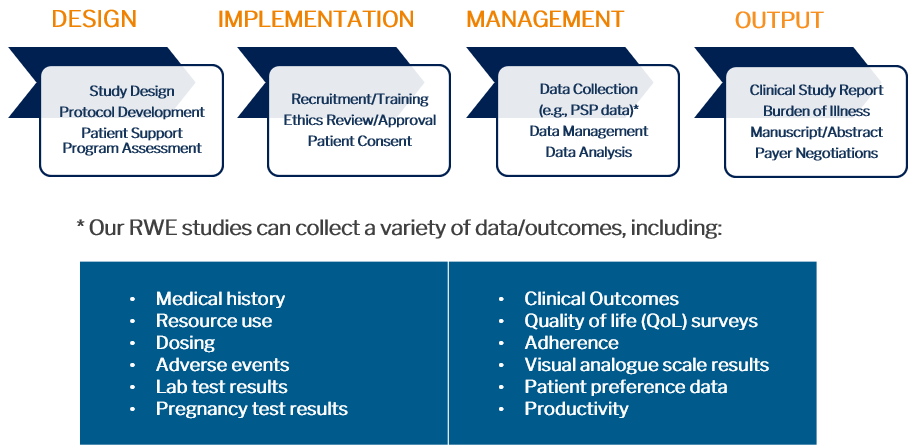
- Design RWE studies and develop RWE study protocols
- Work collaboratively with McKesson’s Patient Support Program (PSP) team to design PSPs that incorporate RWE studies and/or RWD collection
- Complete retrospective RWE oncology studies utilizing McKesson’s huge EMR database of real-world oncology data in the US (through US Oncology and Ontada)
- Analyze and develop valuable market access insights from PDCI/McKesson RWD databases
- Negotiate outcomes-based agreements (OBAs) utilizing RWD/RWE
- Complete Burden of Disease studies utilizing disease registries, administrative databases, and/or published literature
- Prepare abstracts/posters/manuscripts for publication of RWE studies
Figure 1. RWD/RWE Offerings
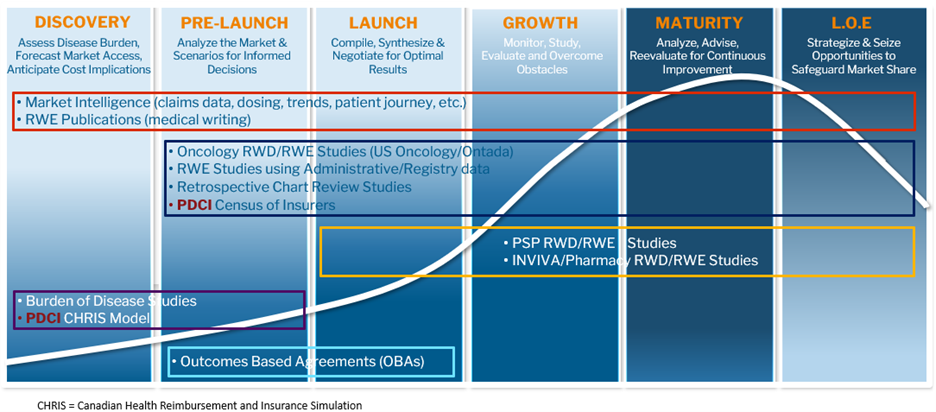
Figure 2. RWE Study Offerings