Each month the pCPA issues an update on the previous month’s negotiation activity. Below, you will find an overview of the new pCPA activity along with PDCI insights into recent developments for your interest.
| Summary of New pCPA Activity Since Last Update | ||
| Category | New Files | Total Files |
|
Active Negotiations
|
4
|
|
| Completed Negotiations (with LOI) | 2 | 297 |
| Completed with No Agreement
(with no LOI) |
0 | 48 |
| No Negotiations | 0 | 73 |
| Considerations at P/T | 0 | 13 |
PDCI TARGET INSIGHTS (Since Last pCPA Update)
Active Negotiations:
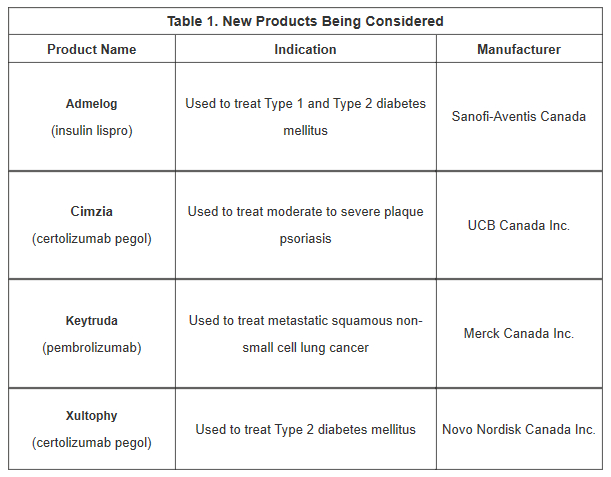
- 4 Products entered active negotiations:
- ADMELOG and XULTOPHY, both products used in treating diabetes mellitus:
- ADMELOG is a biosimilar to Humalog for treatment of Type 2 diabetes.
- XULTOPHY is a biologic combination medication for the treatment of Type 1 and Type 2 diabetes.
- CIMZIA, used to treat moderate to severe plaque psoriasis, is negotiating after receiving its CDR recommendation in November 2019.
- KEYTRUDA is negotiating a new indication in metastatic non-squamous non-small cell lung cancer.
- ADMELOG and XULTOPHY, both products used in treating diabetes mellitus:
Completed Negotiations:
- 2 Products completed negotiations with an LOI:
- BRINEURA, a biologic to treat neuronal ceroid lipofuscinosis Type 2, completed negotiations after 10 months. BRINEURA had entered negotiations based on INESSS recommendation prior to CADTH.
- FOLOTYN, an oncology medication to treat peripheral T-cell lymphoma, completed negotiations after 7 months.
- In January 2020, no products completed pCPA negotiations with no agreement.
PDCI TARGET TRENDS
Stay posted PDCI Target Trends coming in the next pCPA update.
To find out more information about pCPA negotiations and how our team can help with navigating through pCPA discussions, please contact Kaitlyn Proulx at kaitlyn.proulx@pdci.ca.