Each month the pCPA issues an update on the previous month’s negotiation activity. Below, you will find an overview of the new pCPA activity along with PDCI insights into recent developments for your interest.
|
SUMMARY OF NEW PCPA ACTIVITY SINCE AUGUST 31, 2019 |
||
| Category | New Files | Total Files |
| Active Negotiations | 8 | 48 |
| Completed Negotiations (with LOI) | 6 | 276 |
| Completed with No Agreement
(with no LOI) |
1 | 45 |
| No Negotiations | 1 | 69 |
| Consideration at P/T Level | 0 | 13 |
PDCI TARGET INSIGHTS (Since Last pCPA Update)
Active Negotiations:
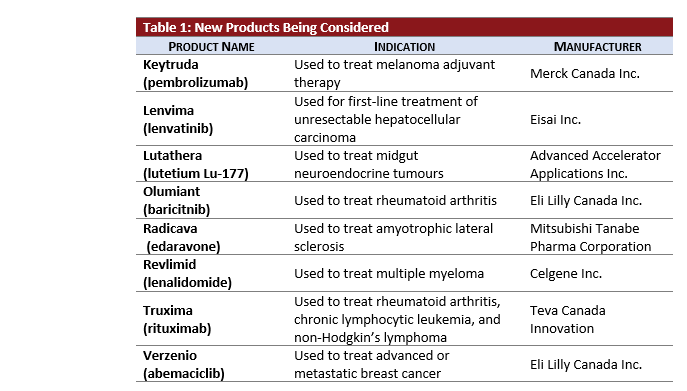
- Five active oncology products, KEYTRUDA, LENVIMA, RADICAVA, REVLIMID, TRUXIMA, and VERZENIO, are newly under pCPA review.
- 40% of all current active pCPA negotiations are oncology products.
- TRUXIMA is under review under the new pCPA biosimilar process.
- KEYTRUDA is now under review for its third active indication for Hodgkin’s Lymphoma.
Completed Negotiations:
- DUPIXENT’s LOI follows INESSS’ notice to the minister in April 2018 to not list unless economic factors were addressed. Originally, CADTH issued a do not reimburse recommendation. A new CADTH submission was received on October 22nd 2019 with specific requested criteria.
- BLINCYTO 4th indication for leukemia closed after being under negotiations for three months.
- OPDIVO 5th indication completed after being under negotiations for almost a year.
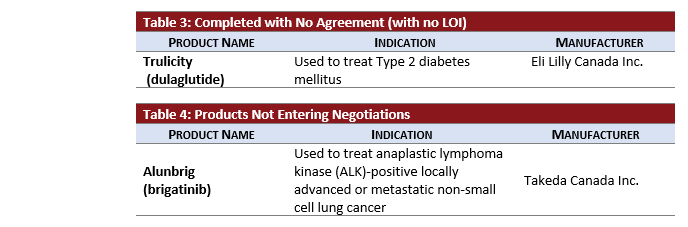
- TRULICITY completed negotiations without reaching an LOI after being under active negotiations for almost two years.
Other Considerations:
- ALUNBRIG did not undergo negotiations after receiving a negative CADTH recommendation.
PDCI TARGET pCPA TRENDS
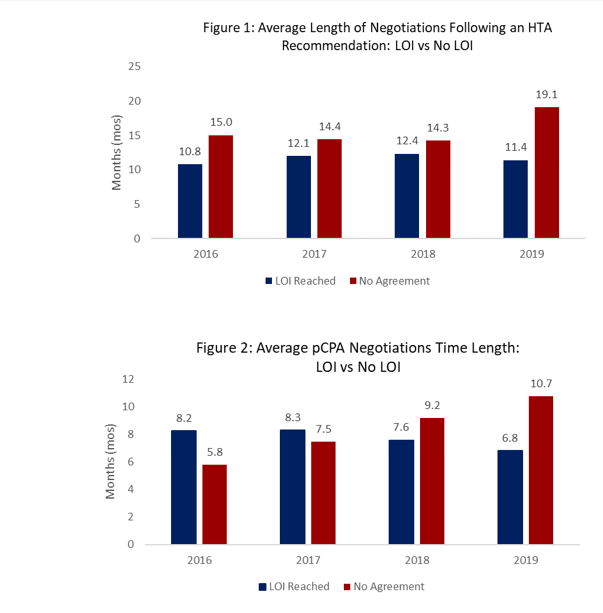
In 2019, there has been a modest reduction in time to LOI. Trends for completed negotiations without LOI are increasing in length.
To find out more information about pCPA negotiations and how PDCI’s reimbursement strategy team can help navigate through pCPA discussions, please contact John-Paul Dowson at jp.dowson@pdci.ca