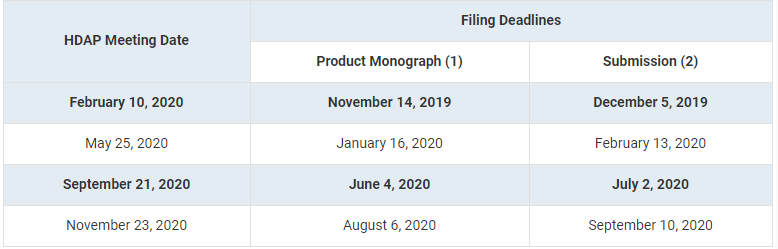
The PMPRB has announced the 2020 meeting dates to the Human Drug Advisory Panel (HDAP)’s , releasing a total of four (4) dates, as per previous years.
This corrected version of TargetPharma makes a distinction between the deadline for submitting the Product Monograph and the deadline for the Patentee’s submission.
- One (1) copy of product monograph or information similar to that included in a product monograph (if product has not yet been approved for sale in Canada) and the proposed level of therapeutic improvement
- One (1) electronic copy of patentee submission
For more information regarding the HDAP meeting schedule and filing requirements, please visit the PMPRB website.
If you are looking for assistance with HDAP submissions for 2020, please reach out to Kimberly Robinson, Director, Pricing and Market Accesss at kimberly.robinson@pdci.ca.