Following the December 2, 2017 publication of proposed amendments to the Patented Medicines Regulations in Canada Gazette Part I, and the corresponding publication of the Regulatory Impact Assessment Statement, PMPRB has published a Guidelines Scoping Paper providing “…an outline of the PMPRB’s preliminary thoughts on how best to operationalize the proposed changes to the Regulations, through non-binding Guidelines…”.
The New Framework would have 5 parts: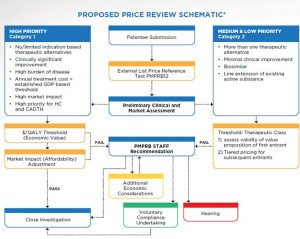
- Interim International Price Reference Test: Introductory price test based on new PMPRB12 basket of reference countries. Products with Canadian prices exceeding the median would be considered potentially excessive.
- Screening: Classification of drugs as either high or low priority based on anticipated impact on Canadian consumers.
- High Priority Drugs: Assess $/QALY against explicit threshold & expected impact on payers within first 3-5 years.
- Medium & Low Priority Drugs: PMPRB could employ revised Therapeutic Class Comparison requiring each successive entrant to reduce price from preceding entrant.
- Re-benching: Periodic re-benching to ensure previous price ceilings or determinations of excessive pricing.
Source: PMPRB Guidelines Scoping Paper – High Level Overview of Potential New Framework
Though details remain to be worked through consultation processes, PMPRB indicates that Guideline reform will be its focus for upcoming annual outreach sessions for patentees in January 2018, and is anticipating a public first draft in Spring 2018.
Please see the more information on the PMPRB website.
